To understand the chapter hydrocarbons you have to study the chapter. Email protected email protected.
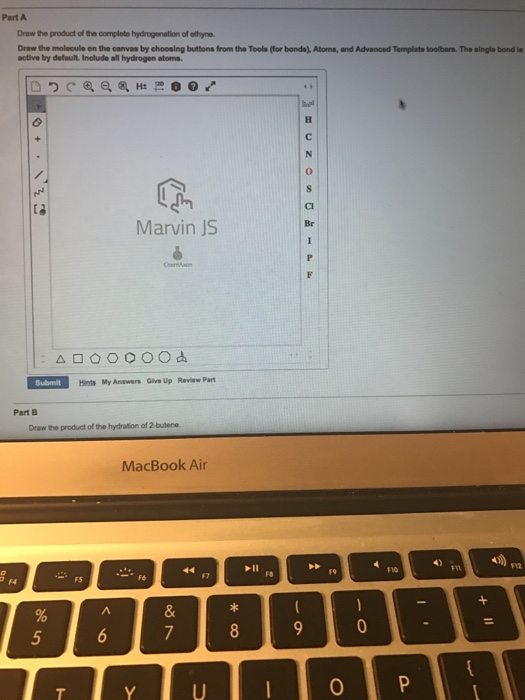
Solved Draw The Product Of Completo Hydrogenation Of Ethyne Chegg Com
IUPAC name of the first member of the series.
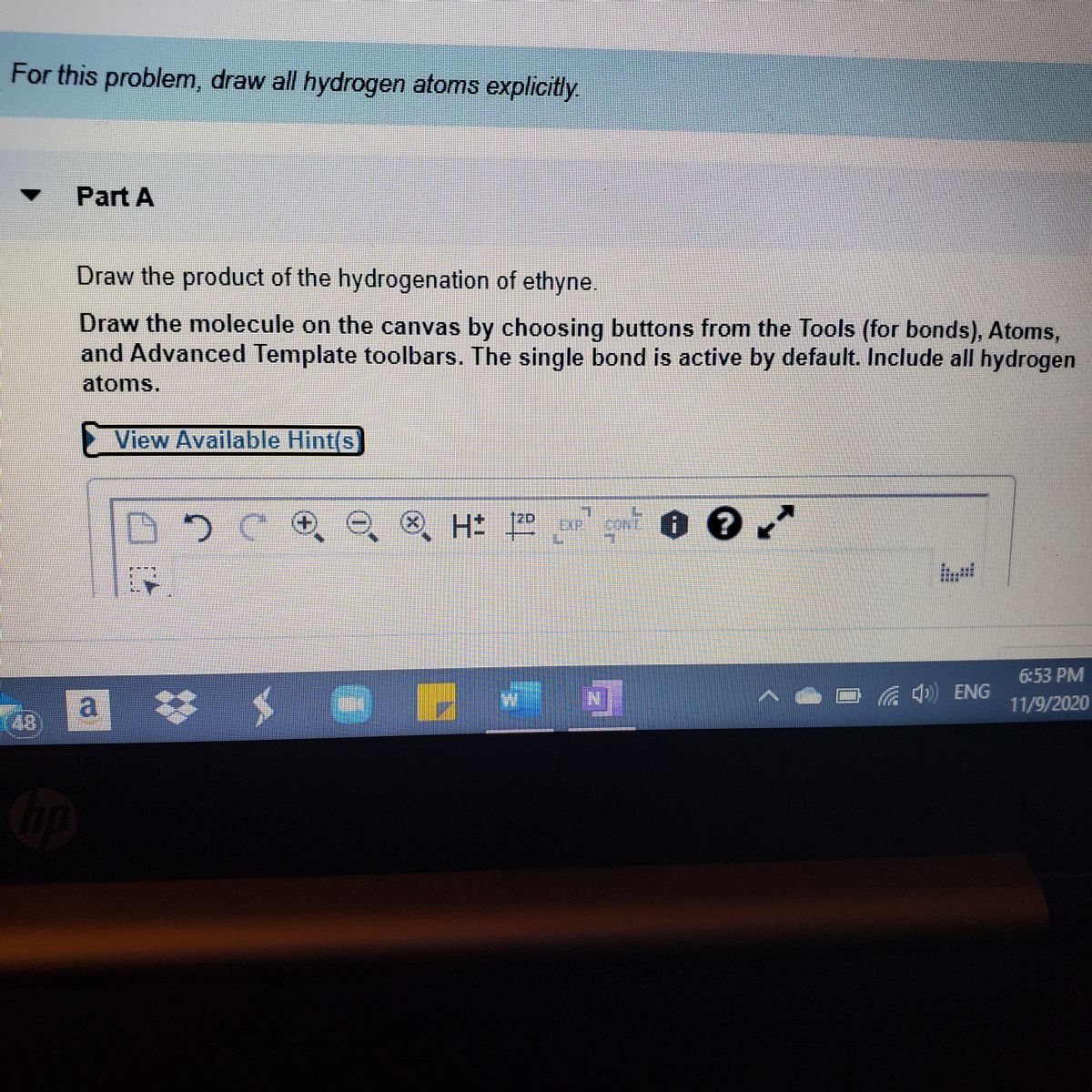
. 17 Name the reagents for the complete hydroboration-oxidation reaction in step 1 and step 2. C n H 2n. Ethyne In the above structure both carbons are bonded.
16 Which rule is obeyed by hydroboration oxidation process. The viability of catalyst recovery has been demonstrated in three different systems including in two cases where 99 ee can be achieved under recycling conditions up. C n H 2n2.
Cationic Rh complexes of these ligands have been prepared in a separate step and they have been found to be excellent catalysts for organic and aqueous phase hydrogenation of dehydroaminoacids. When methane is burnt in excess of air or oxygen with pale blue flame it gives carbon dioxide gas water and heat energy. At 500 K the equilibrium constant for.
D State any two uses of diamond. Ethanol is manufactured by the hydrogenation of acetaldehyde according to the reaction. Your browser will take you to a Web page URL associated with that DOI name.
Type of reaction with chlorine. Characteristics bond type. In pure oxygen ethyne undergoes complete combustion and high temperature suitable for welding is attained.
Ii reduction via catalytic hydrogenation catalytic addition of hydrogen. Draw a simple diagram to show the arrangement of carbon atoms in diamond. Send questions or comments to doi.
Copy and complete the following table which relates to three homologous series of hydrocarbons. B A diamond crystal is a giant molecule of carbon atoms. A Diamond is a colourless transparent substance having extraordinary brilliance.
It is made up of carbon. This reaction is complete oxidiation reaction. Whereas air contains less percentage.
It has been used by more than 2 million students. In a hydrogenation reaction the final product is the saturated. See also 8j iii oxidation by cold alkaline solution of manganateVII ions to form the diol iv oxidation by hot acidified solution of manganateVII ions leading to the rupture of the carbon-to-carbon double bond in order to determine the position of alkene linkages in larger molecules.
IUPAC name of the homologous series. Draw the electron-dot structure for ethyne. Eg ethene reacts with hydrogen to give ethane This reaction is also called saturation of the double bond.
It really has been that long. Assume ideal gases and for the given reaction ΔG-4027 kJmol at 650 K P1 bar. C Explain why diamond has a high melting point.
Each carbon atom in the diamond crystal. Type or paste a DOI name into the text box. E Conversion of Methane to Ethyne C 2 H 2 When methane is heated to about 1500C in an electric arc and then suddenly cooled the product is C 2 H 2 and Hydrogen.
In your opinion why cannot we use a mixture of ethyne and air for this purpose. It has been national bestseller for more than 65 years. A nickel catalyst is also needed to accomplish this addition reaction.
The rule obeyed by hydroboration oxidation process is opposite to the Markovnikovs rule. Preface The Essentials of Physical Chemistry has been written for BSc students. In a Hydrogenation reaction hydrogen H 2 is added across the double bond converting an unsaturated molecule into a saturated molecule.
In ethene the carbon atoms are said to be unsaturated. The complete and accurate NCERT Solutions for Class 11 Chemistry Chapter 13 will be updated soon Download NCERT Solutions Class 11 Chemistry Chapter 13 PDF Organic chemistry is the scientific study of the structure properties composition reactions and synthesis of organic compounds. A mixture of ethyne and oxygen is burnt for welding.
And if no other reaction occurs what is the mole fraction of CH3CHO of the product gas from the reaction. It is 26 editions old. The product formed in the reaction of reverse of dehydration of alcohol is alkene.
Alkenes may be turned into alkanes by reacting the alkene with hydrogen gas at a high temperature and high pressure. C n H 2n-2. Note that the word hydrogen is found in this reaction name making it easier to remember and recognize.
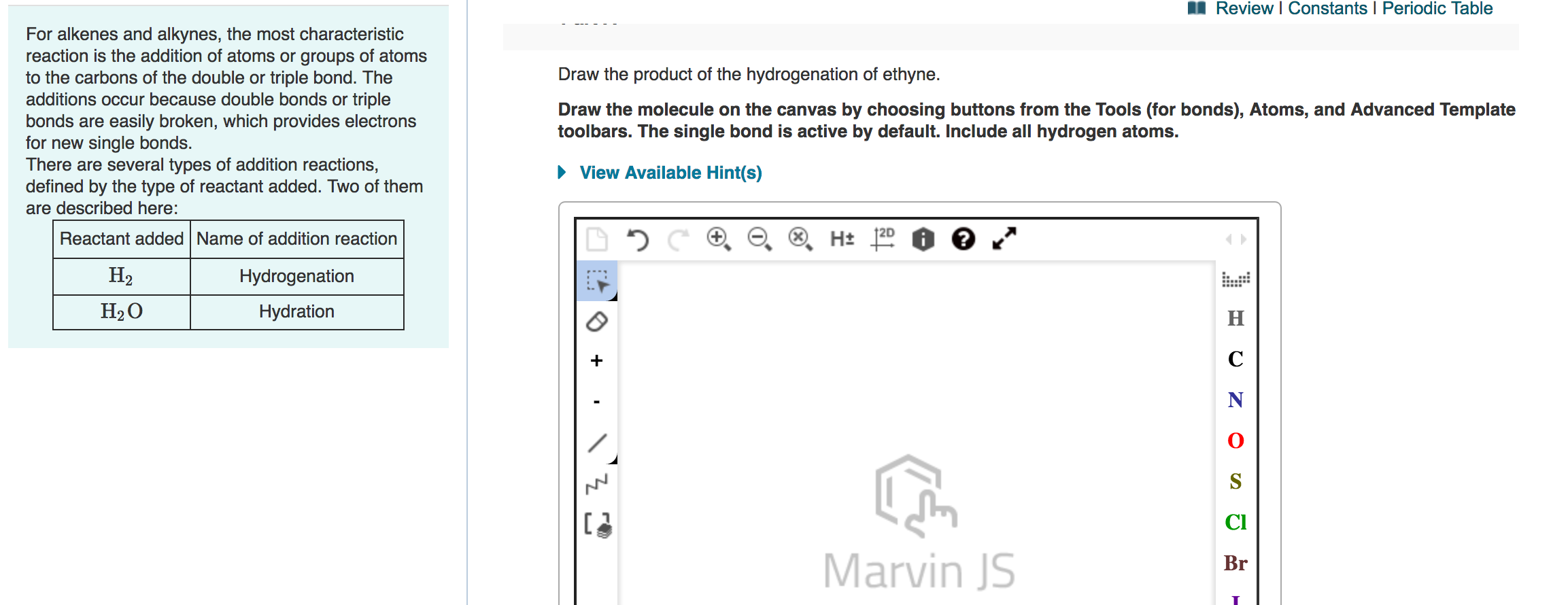
Solved A Review Constants Periodic Table Draw Chegg Com
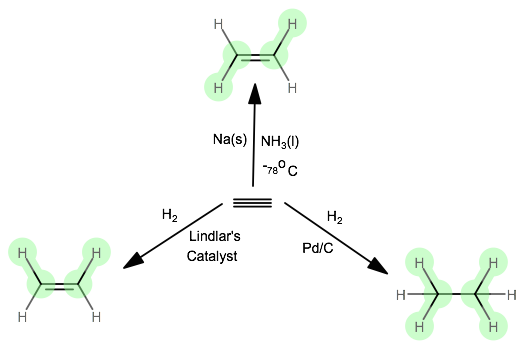
How Would You Draw The Product Of The Hydrogenation Of Ethyne Socratic
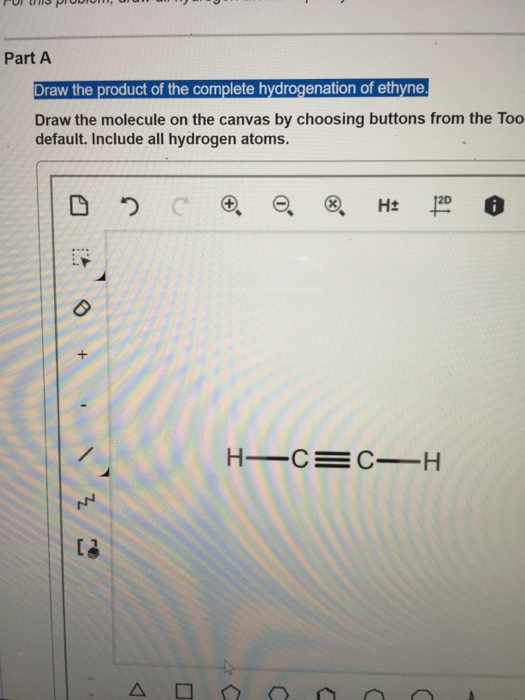
Solved Draw The Product Of The Complete Hydrogenation Of Chegg Com
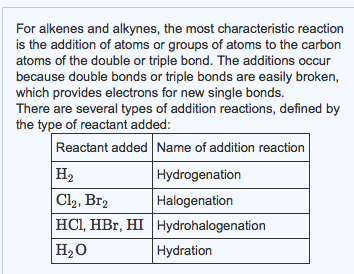
Solved 1 Draw The Product Of The Complete Hydrogenation Of Chegg Com

Complete Hydrogenation Of Ethyne To Ethane Conversion Of Ethyne To Ethane Youtube

Answered Draw The Product Of The Hydrogenation Bartleby

Answered Draw The Product Of The Hydrogenation Bartleby

Solved 10 Of 13 Review I Constants 1 Periodic Table Part A Chegg Com
0 comments
Post a Comment